COMUNICACIONES BREVES
Detection of JAK2 V617F mutation in Philadelphia chromosome-negative myeloproliferative neoplasms
Detección de la mutación JAK2 V617F en neoplasias mieloproliferativas cromosoma Philadelphia negativas
Santos Cruz AlemánI, Adriana Larios RoqueI, Uxmal Caldera SuazoI , Carlos Santamaria QuesadaII, Ann-Christin Puller I, Allan Pernudy UbauI
I
Molecular Biology Laboratory 'M.A. Elmer Cisneros in Memoriam', Polytechnic
Institute of Health, National Autonomous University of Nicaragua (UNAN). Managua,
Nicaragua.
II Clinical
Laboratory Department, Molecular Biology Laboratory, National Children's Hospital.
San José, Costa Rica.
ABSTRACT
Introduction:
Myeloproliferative neoplasms (MPNs) comprise several hematological neoplasms
such as polycythemia vera (PV), essential thrombocythemia (ET), and idiopathic
myelofibrosis (IMF). Molecular lesions have been investigated as the underlying
mechanism for the genesis of MPNs with the specific mutation in the Janus kinase
2 (JAK2) gene, JAK2 V617F, being most frequent. The variant JAK2 V617F
protein is characterized by a substitution of valine for phenylalanine at position
617 (V617F) resulting in constitutively unregulated enzymatic activity.
Objectives: Currently, the diagnoses of MPNs in Nicaragua are based solely
on clinical presentation of the patient and routine blood analyses. We aim for
the implementation of the molecular diagnosis of JAK2 V617F in order to allow
for a more specific classification and to provide targeted therapy for positive
patients.
Methods: To detect the JAK2 V617F mutation in MPNs patients, a cross-sectional
study was performed with 29 patients with clinical diagnosis of PV, ET, or IMF.
DNA was extracted from whole peripheral blood using a commercial kit. A polymerase
chain reaction (PCR) technique was carried out to detect the mutation at the
genomic level using allele-specific primers.
Results: This study represents the first report of JAK2 V617F analysis
in Nicaragua. The mutation was detected in 14 out of 29 MPNS patients. Following
the recommendations of the World Health Organization (2016), we suggest the
molecular testing for the JAK2 V617F mutation as part of the initial diagnostic
portfolio for patients suffering myeloproliferative neoplasms in Nicaragua and
in the rest of Latin American countries.
Keywords: JAK2 V617F, endpoint PCR, myeloproliferative neoplasms.
RESUMEN
Introducción:
las neoplasias mieloproliferativas (NMP) comprenden varias neoplasias hematológicas
como la policitemia vera (PV), la trombocitemia esencial (TE) y la mielofibrosis
idiopática (MI). Lesiones moleculares han sido investigadas como el mecanismo
subyacente para la génesis de las NMP con la mutación específica
en el gen Janus kinasa 2 (JAK2), siendo la mutación JAK2 V617F la más
frecuente. La variante de la proteína JAK2 V617F se caracteriza por una
sustitución de valina por fenilalanina en la posición 617 (V617F)
dando como resultado una actividad enzimática constitutivamente no regulada.
En la actualidad, los diagnósticos de NMP en Nicaragua se basan únicamente
en la presentación clínica del paciente y análisis de sangre
de rutina. El objetivo de este trabajo es la implementación del diagnóstico
molecular de JAK2 V617F con el fin de permitir una clasificación más
específica y proporcionar terapia dirigida a pacientes positivos.
Métodos: para detectar la mutación JAK2 V617F en pacientes
con NMP, se realizó un estudio transversal con 29 pacientes con diagnóstico
clínico de PV, TE o MI. El ADN se extrajo de sangre periférica usando
un Kit comercial. Se llevó a cabo una técnica de reacción en
cadena de la polimerasa (PCR) para detectar la mutación a nivel genómico
usando cebadores alelos específicos.
Resultados: este estudio representa el primer informe del análisis
JAK2 V617F en Nicaragua. La mutación se detectó en 14 de los 29 pacientes
con NMP. Siguiendo las recomendaciones de la Organización Mundial de la
Salud (2016), se sugiere la prueba molecular de la mutación JAK2 V617F
como parte de la cartera diagnóstica inicial para pacientes con neoplasias
mieloproliferativas en Nicaragua y en el resto de países latinoamericanos.
Palabras clave: JAK2 V617F, PCR punto final, neoplasias mieloproliferativas.
INTRODUCTION
Philadelphia chromosome-negative myeloproliferative neoplasms (MPNs) include several clonal hematological neoplasms, such as polycythemia vera (PV), essential thrombocythemia (ET) and idiopathic myelofibrosis (IMF), which are thought to arise from the transformation of a hematopoietic stem cell. The main clinical features of these diseases are overproduction of functional mature blood cells and a protracted clinical course.1 The use of molecular techniques has allowed to uncover the pathogenesis of these neoplasms. Mutations in Janus kinase 2 (JAK2) have been identified that result in the constitutive activation of its kinase activity, thereby elevating the proliferative capacity and survival of the mutated cells. 2 In 2005, Jones and colleagues conducted a study with 480 patients diagnosed with MPNs, finding that 81% of patients diagnosed with PV and 41 % of ET patients carried the JAK2 V617F mutation.3 More recent reports confirm JAK2 V617F presence in 95 % of PV patients and 50-60 % of ET and IMF patients.4 Thus, the detection of this JAK2 mutation is a pivotal diagnostic feature recognized in the World Health Organization (WHO) classification for MPNs since 2008, alongside the measurement of serum erythropoietin.6 Furthermore, constitutively activated JAK2 kinase can be targeted by therapeutic inhibition, emphasizing the relevance of analyzing the presence of JAK2 mutations.7
In Nicaragua, the detection of the JAK2 V617F mutation is not yet being performed. Our aim is the implementation of the PCR-based JAK2 V617F analysis for the first time in Nicaragua. The identification of JAK2 V617F-positive patients will have a strong impact on the diagnosis, classification, and treatment of MPNs.
METHODS
The study was conducted in compliance with the ethical regulations and principles governing research.
A cross-sectional study was performed with 29 patients with clinical diagnosis of MPNs. Samples were obtained from the hematology services of the national referral hospital Teaching Hospital "Roberto Calderón" and the therapeutic phlebotomy service ( Blood Bank) of the Nicaraguan Red Cross in order to detect JAK2 V617F.
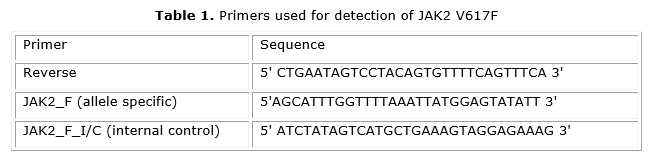
A PCR assay for JAK2 V617F detection was implemented for the first time by Baxter and colleagues in 2005 in the United Kingdom.5 The implementation of this technique was carried out in the Molecular Biology Laboratory of the Polytechnic Institute of Health, POLISAL, UNAN-Managua. DNA was extracted from 200 mL of whole peripheral blood in EDTA as anticoagulant using a commercial kit (QIAamp DNA Blood Mini Kit) following the manufacturer's recommendations. PCR experiments were conducted as previously described.5 This PCR uses two forward primers, called JAK2_F and JAK2_F_I/C and one common reverse primer (JAK2_R). The JAK2_F primer is specific for the mutant allele while the JAK2_F_I/C primer amplifies both the normal and the mutant allele, thus serving as an internal control of the PCR reaction (table).
RESULTS
Twenty-nine cases of MPNs were analyzed by allele-specific PCR including 18 patients with PV, ten with ET, and one patient with IMF. Of the 29 investigated cases, 14 were carriers of the JAK2 V617F mutation. Eleven presumptive PV patients were positive (61 %), as well as three diagnosed as ET patients (30 %). The only investigated IMF case was PCR-negative.
Figure depicts the electrophoresis of JAK2V617F-specific PCR amplicons for four PV patients of which two were positive for the mutation. We therefore demonstrate the successful establishment of the PCR-based JAK2 V617F detection in Nicaragua.
DISCUSSION
The JAK2 V617F mutation was detected in approximately half of the analyzed 29 patients (14/29) with MPNs. Interestingly, only 61 % of the presumptive PV cases were PCR-positive although all presented clinical traits and even laboratory data suggesting the presence of PV. In the remaining cases, the presence of a related syndrome cannot be excluded. Especially in potential cases of PV which are PCR-negative for JAK2 V617F further studies of the JAK2 gene should be indicated. For example, mutations affecting exon 12 of the JAK2 gene were described, which range from point mutations to small insertions or deletions.8 Most of these alterations are associated with PV, however, they only account for 4 % of the cases. It is also valid to indicate the possibility of a secondary polycythemia which shares clinical symptoms with primary polycythemia but shows increased erythropoietin levels and is negative for JAK2 mutations.8,9 The analysis of potential mutations in JAK2 exon 12 and of erythropoietin levels will be addressed in future investigations. The importance of discriminating primary and secondary polycythemia is that patients with the secondary form do not need cancer treatment such as hydroxyurea which entails a number of side effects and might have a negative impact on the patients´ quality of life. Detection of JAK2 V617F in patients with PV is relevant as targeted therapies directly inhibiting JAK2 kinase activity, such as ruxolitinib, dasatinib or nilotinib, result in minimal myelosuppression and reduced secondary toxicity.10
This study of the detection of the JAK2 V617F mutation in patients with clinical diagnosis of MPNs represents the first report of this kind in Nicaragua. In accordance with the WHO recommendations, the PCR-based analysis of JAK2 V617F can now be incorporated as a primary diagnostic test for patients with presumptive MPNs in this country.
Incorporating molecular techniques that facilitate medical diagnosis in the field of hematology is of great scientific value for the Polytechnic Institute of Health at the UNAN-Managua and will immediately influence the quality of the patients´ life.
ACKNOWLEDGMENT
We express our gratitude to MSc. Martha Delgado Guerrero, Polytechnic Institute of Health, UNAN, Managua; MD Douglas Rosales, Hematology Service Teaching Hospital "Roberto Calderon"; MD Rene Berrios, National Blood Program of the Nicaraguan Red Cross, for their support in this research.
Financial sources: Funds for research projects (FPI), UNAN, Managua.
Conflict of interest: The authors declare no conflict of interest.
REFERENCES
1. Spivak JL. Myeloproliferative Neoplasms. N Engl J Med 2017; 376(22):2168-81. DOI: 10.1056/NEJMra1406186
2. Navarro-Vázquez M. Marcadores moleculares de los síndromes mieloproliferativos. Rev Hematol Mex 2010;11(3):152-5.
3. Jones AV, Kreil S, Zoi K, Waghorn K, Curtis C, Zhang L, et al. Widespread occurrence of the JAK2 V617F mutation in myeloproliferative disorders. Blood 2005; 106(6):2162-8.
4. Cross NC. Genetic and epigenetic complexity in myeloproliferative neoplasms. Hematology Am Soc Hematol Educ Program. 2011; 2011:208-14.
5. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau M, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016; 127(20):2391-2405. DOI 10.1182/blood-2016-03-643544.
6. Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005; 365:1054-61. DOI: 10.1016/S0140-6736(05)71142-9
7. Bose P, Verstovsek S. JAK2 inhibitors for myeloproliferative neoplasms: what is next?. Blood. 2017; 130(2): 115-125. DOI:10.1182/blood-2017-04-742288
8. Scott LM, Tong W, Levine RL, Scott MA, Beer PA, Stratton MR, Futreal PA, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 2007; 356:459-68.
9. Besses, C.; Cervantes, F. Manual de Recomendaciones en Neoplasias Mieloproliferativas Crónicas Filadelfia Negativas. Grupo Español de Neoplasias Mieloproliferativas Filadelfia Negativas (GEMFIN), 2014.
10. Geyer H, Tibes R, Mesa, R. JAK2 inhibition: current roles in myelofibrosis and initial lessons learned from Mexico. Hematología. 2013;14(1):26-36.
Recibido: junio
09, 2017.
Aceptado: septiembre
18, 2017.
Dr. Allan Pernudy
Ubau . Molecular Biology Laboratory 'M.A. Elmer Cisneros in Memoriam', Polytechnic
Institute of Health, National Autonomous University of Nicaragua (UNAN), Recinto
Universitario 'Ruben Dario', de la Rotonda Universitaria 1 km al Sur, Managua,
Nicaragua;
Tel: +505-2277-0267
ext. -5197. Email: pernudi@gmail.com.